



L. Thalund Instrument ApS
Retails sterile packaging, chemical indicators and sealing devices for the Industry and health sector, as well as medicine dosing systems for the health sector from Europe's leading suppliers.
Today, the company appears as a strong trade partner due to a structured business organization, stable finances, good agencies and service-minded employees. This gives our customers a guarantee that we will also be a safe, stable and healthy trading partner in the future.
Strategy and visions
- Offer quality products at competitive prices.
- To obtain exclusive distribution on the latest products.
- To offer the best and fastest service.
-
L. Thalund Instrument ApS
established in 1982, sells sterile packaging, chemical indicators, and sealing devices for the Industry and health sector, as well as medicine dosing systems for the health sector from Europe's leading suppliers.
Today, the company appears as a strong trading partner due to a structured business organization, stable finances, good agencies and service-minded employees. This gives our customers a guarantee that we will also be a safe, stable and healthy trading partner in the future.
-
Marketing
We have placed great emphasis on maintaining close relationships with our customers and suppliers. This is the reason why the majority of our marketing takes place via close telephone customer contact and email correspondence.
Through this form of marketing, the customer benefits from competitive prices, products, services and our international network.
This also gives us the opportunity to continuously keep our customers informed and up-to-date on new products, qualities and possibly price reductions. -
Personnel policy
The focal point in L. Thalund Instrument ApS is the staff and the individual employee. Right from the start, it was known that the core and essence of any streamlined and modern organization are happy and satisfied employees.
In addition, we do a lot to ensure that the employees are in close dialogue with suppliers and manufacturers, which is why they regularly participate in fairs, courses and conferences at home and abroad.
Latest product from L. Thalund Instrument
The perfect weekly box for medication dosing at home and on the go.
Wiegand has developed a new weekly medication system that meets your needs for taking medication at home or on the go.
The WiBox pro medicine box provides a fast, easy and safe way to dose and handle medicine.
You have an overview of your medication care for a whole week using WiBox pro. A single box can be taken with you, for a day, or the whole box when you go travelling.
Validation
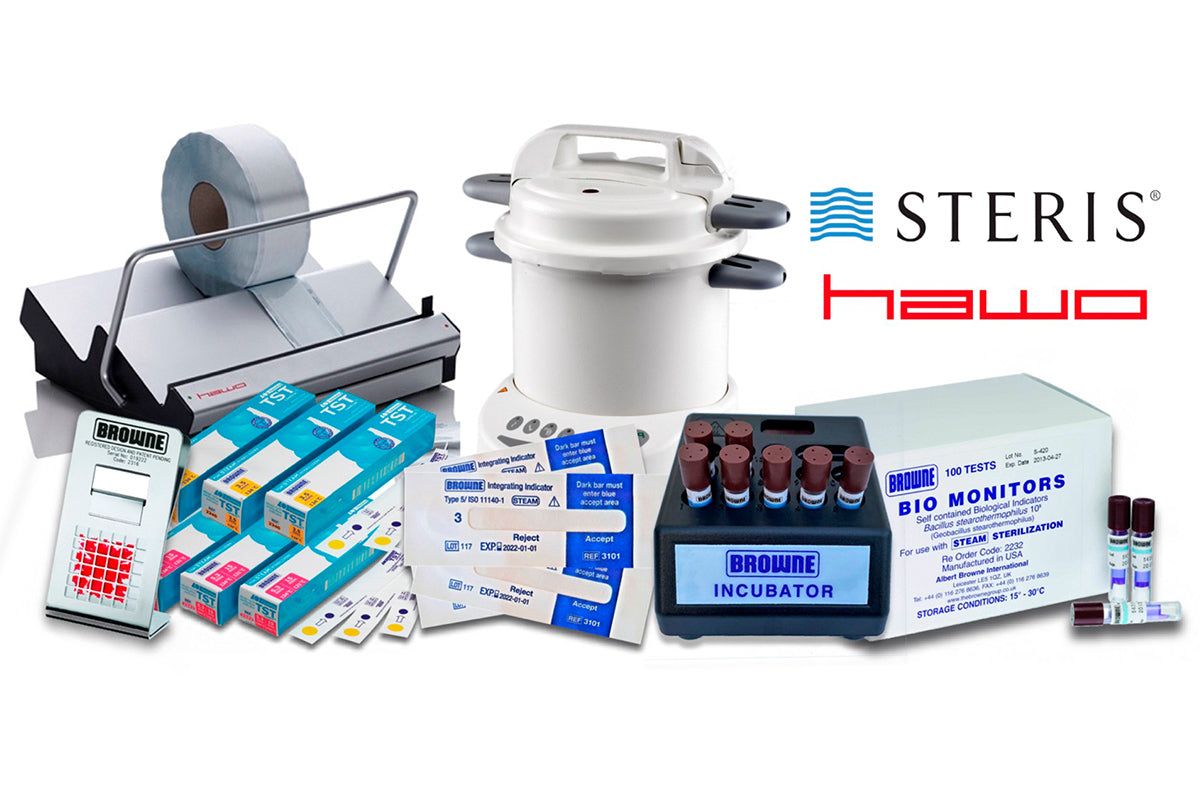
Validation of sealing devices and packaging.
We carry out validation of all sealing devices regardless of make. This is carried out according to the standards in ISO 11 607 and EN 868 – 2009. When validating the sealing device, the critical process parameters are checked as described in ISO 11 607 – 2: 2006 point 5.6.2. Validation is performed with our test equipment that is calibrated according to the requirements. Certificates for these can be obtained here on the website.
Inspection and testing of the seal is carried out in the following areas:
- Width
- Openings
- Channels
- Cracks
- Material delamination
- Material separation
- Melting
- Withdrawal
The strength is checked according to EN 868 – 5: 2009, point 4.5.1 and annex D:
- "The strength of the seal must be at least 1.5 N per 15 mm width, both before and after the material has been through the sterilization process”.
- 3 tests carried out before sterilization.
- 3 tests carried out after sterilization 121º
- 3 tests carried out after sterilization 134º
The strength is tested using the HT 150 SCD.
The tightness of the seal is checked by "Dye Penetration" carried out in accordance with EN ISO 11607-1, Annex B. The test method described in ASTM F1929 is used."
Autoclave bag with colored liquid. Observe for 20 seconds for penetration of the seal. Documented with a picture.
If you have further questions about validation, you are welcome to contact us.
